Updated Guidelines Approve Self-Swab HPV Testing At Home And In The Office
Published: February 11, 2026l
If you’ve delayed a visit to your OB-GYN because you hate the uncomfortable-though-important Pap test, it’s time to reconsider. New guidelines from the government and the American Cancer Society support self-administered swab tests as a replacement for a Pap test, making HPV testing more accessible and comfortable than ever.
As the chair of the OB/GYN department, I believe this is an important and necessary step to reducing barriers that prevent women from getting care. The option for women to self-collect either at home or in a clinician’s office means that a woman doesn’t have to participate in an exam that is historically so uncomfortable. To me, this is such a logical and valuable step to improve access for women.
Now, the newest guidance for cervical cancer screening from the government’s Health Resources and Services Administration (HRSA) allows for self-collected samples for screening. As a result, private insurance companies are expected to cover the newest options beginning in January 2027.
Not only do the updated guidelines challenge where you need to have the test done, but they also challenge how often women need to be screened. Historically, we believed that you needed to have a Pap test completed every year. Now, the HRSA recommends that testing be done every five years for average-risk women between 30 and 65 years of age.
The reality is that you don’t contract an HPV infection on Monday and then have cervical cancer on Tuesday; there’s a significant lag from exposure to potential development of cancer. You can test less often with the newer options because they screen specifically for the high-risk types of the virus. A Pap test, on the other hand, looks at the cytology (the collection of cells) so any strain of HPV could cause an abnormal result, meaning more testing for the patient.
These encouraging updates mean that HPV testing can be done in a way that is convenient and accessible for women – while still being effective at detecting HPV strains that pose a danger to her health.
As the chair of the OB/GYN department, I believe this is an important and necessary step to reducing barriers that prevent women from getting care. The option for women to self-collect either at home or in a clinician’s office means that a woman doesn’t have to participate in an exam that is historically so uncomfortable. To me, this is such a logical and valuable step to improve access for women.
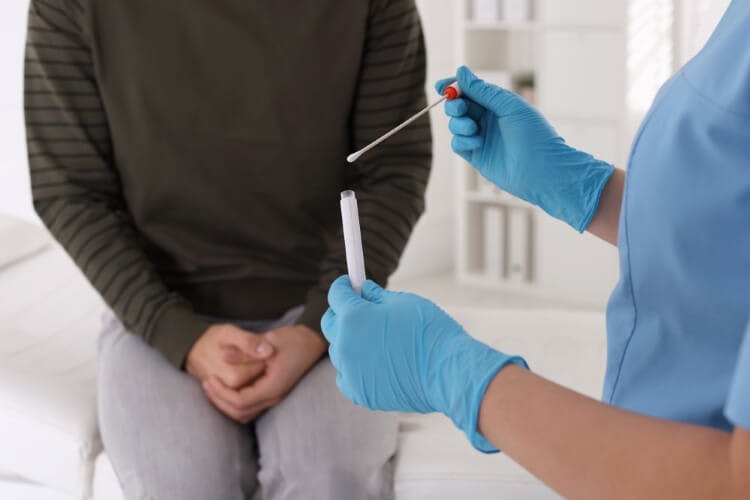
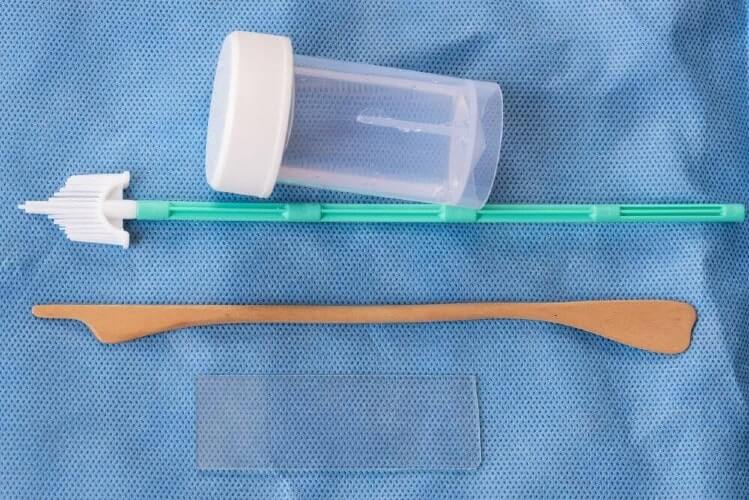
What are the self-collect tests?
In late 2025, the American Cancer Society updated its recommendations to include Food and Drug Administration (FDA)-approved self-swab tests. Two of those options are in-office self-administered tests, and the third is the Teal Wand, which can be administered at home.Now, the newest guidance for cervical cancer screening from the government’s Health Resources and Services Administration (HRSA) allows for self-collected samples for screening. As a result, private insurance companies are expected to cover the newest options beginning in January 2027.
Why are Pap or HPV tests important?
HPV is the most common sexually transmitted infection in the United States, affecting both men and women. There are more than 40 different types of strains, and the screenings target the 13 most dangerous ones that cause cancers, including anal, vulval, oropharyngeal (back of throat), vaginal, and penile. Since HPV screening and prevention began in the mid-1970s, cervical cancer rates have been cut in half.Not only do the updated guidelines challenge where you need to have the test done, but they also challenge how often women need to be screened. Historically, we believed that you needed to have a Pap test completed every year. Now, the HRSA recommends that testing be done every five years for average-risk women between 30 and 65 years of age.
The reality is that you don’t contract an HPV infection on Monday and then have cervical cancer on Tuesday; there’s a significant lag from exposure to potential development of cancer. You can test less often with the newer options because they screen specifically for the high-risk types of the virus. A Pap test, on the other hand, looks at the cytology (the collection of cells) so any strain of HPV could cause an abnormal result, meaning more testing for the patient.
These encouraging updates mean that HPV testing can be done in a way that is convenient and accessible for women – while still being effective at detecting HPV strains that pose a danger to her health.
Featured Expert/ Author

Stamford Health Medical Group Member












)